

The impact of diabetes


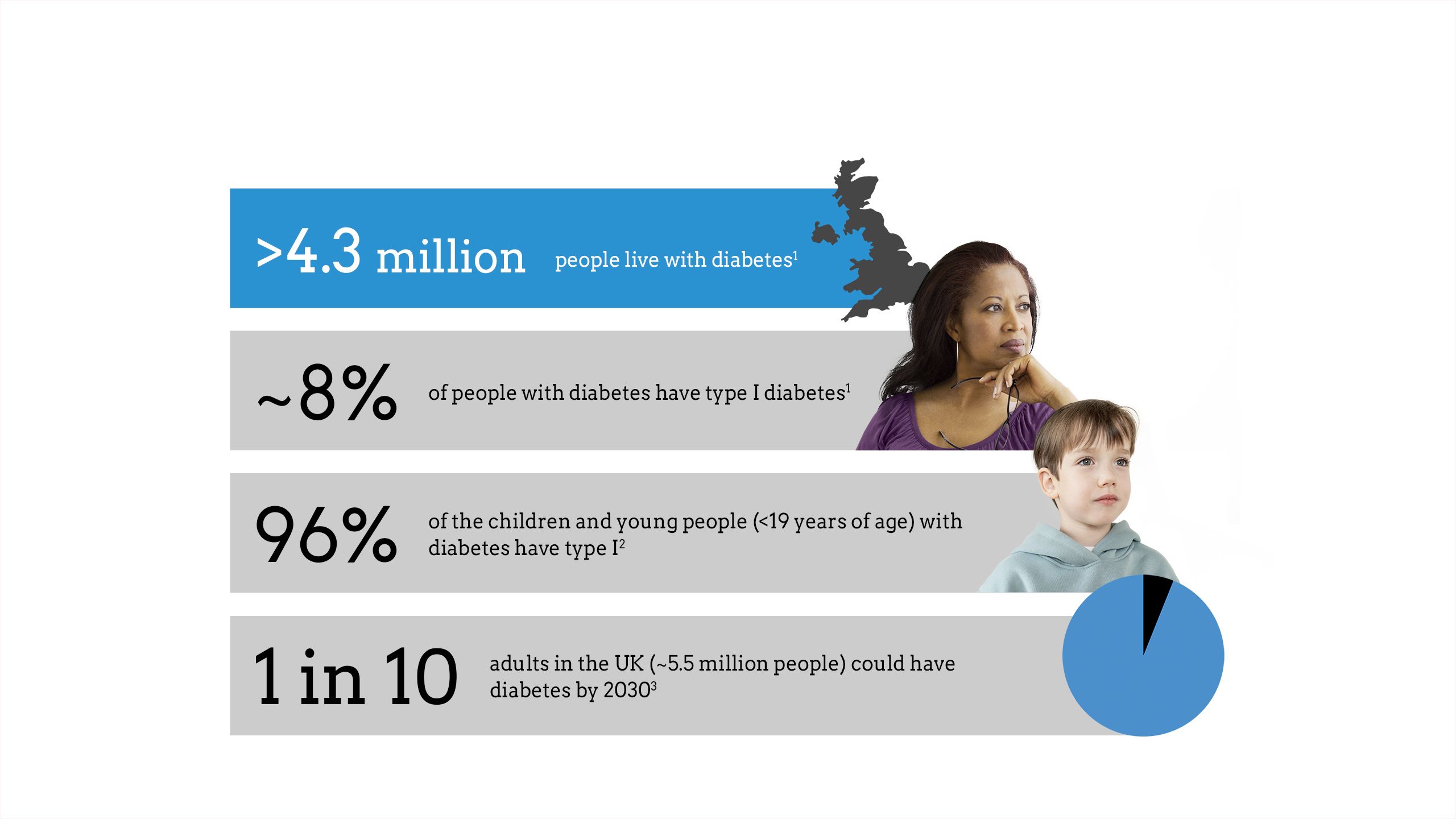
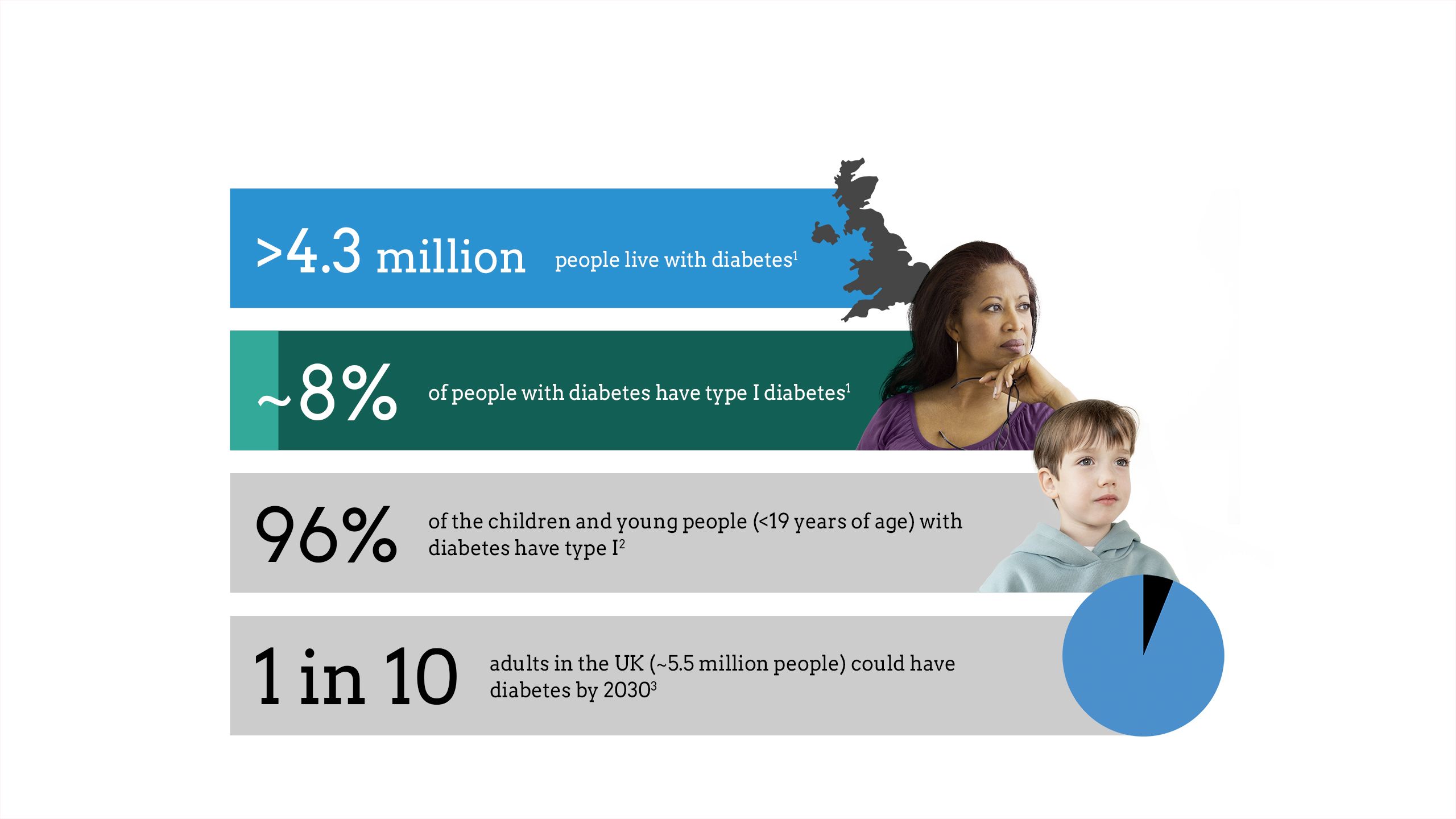
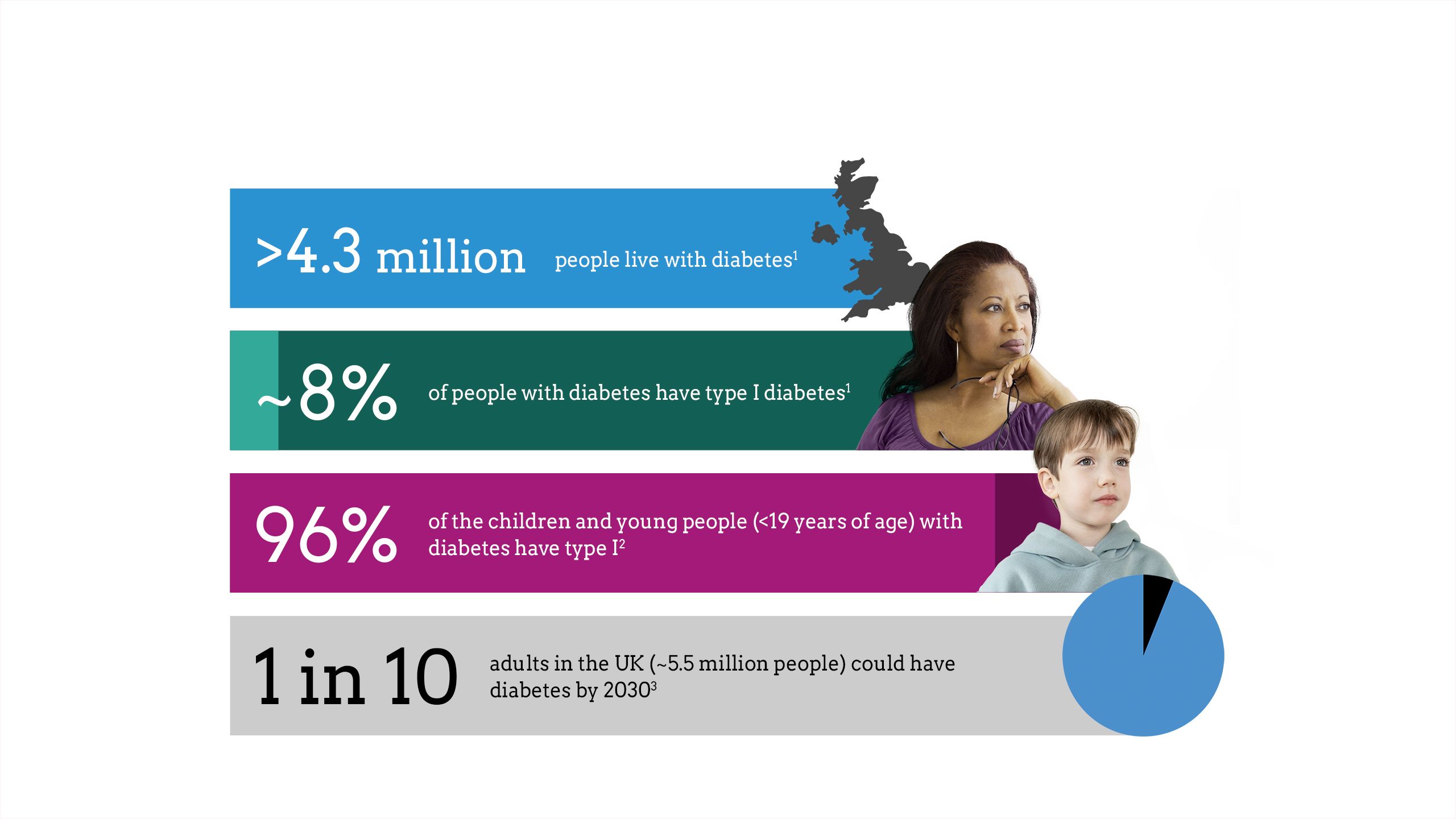
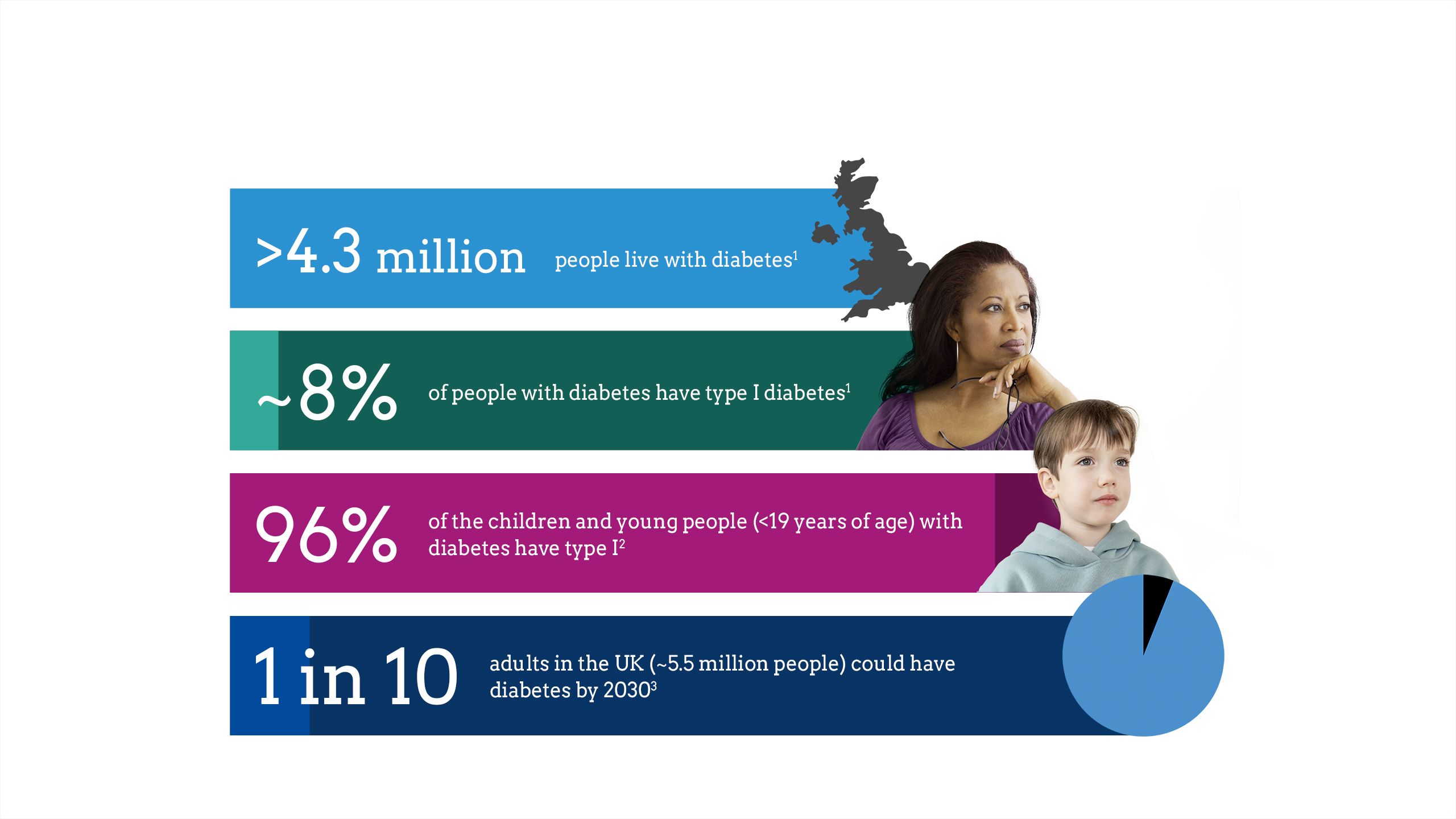
Maintaining glycaemic control is critical to diabetes management
Glycaemic control means maintaining optimal blood glucose concentration in patients with diabetes.4
Glycaemic control is assessed by measuring the amount of blood glucose attached to haemoglobin, known as the haemoglobin A1c (HbA1c) measurement.5 HbA1c tests indicate the average glucose level over 90 days.6
Patients with type I diabetes should aim for a target HbA1c level of 48 mmol/mol (6.5%) or lower.7

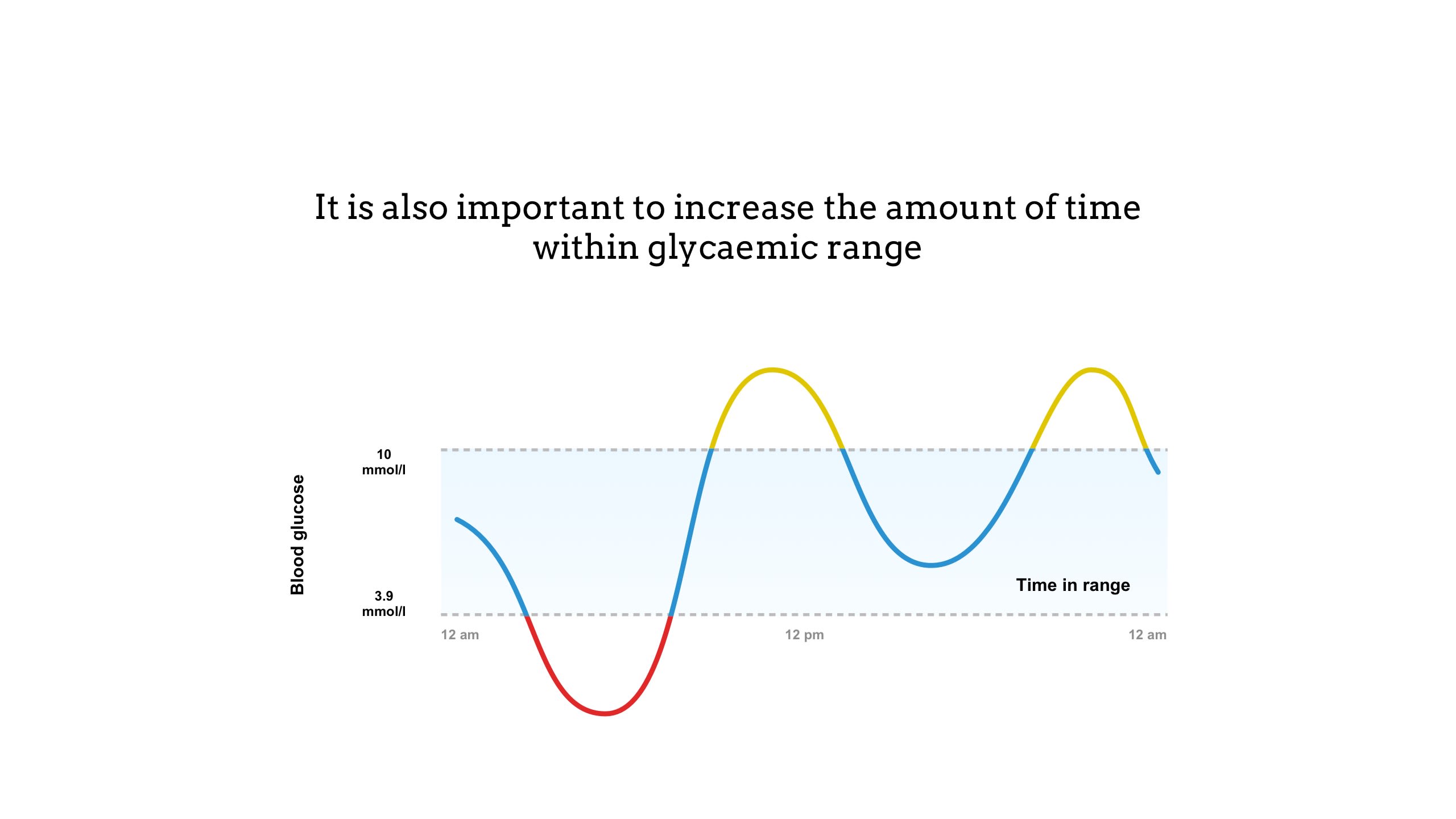
Time in range is a measure of blood glucose control that shows the percentage of time a person spends within a target glucose range (3.9 to 10mmol/l)8
• Time below range (in red above) indicates hypoglycaemia and is when the blood glucose level drops too low, below 3.9mmol/l.9
• Time above range (in yellow above) indicates hyperglycaemia and is when blood glucose levels are too high, usually above 10mmol/l.10
Alongside HbA1c, time in range is an effective way of understanding glycaemic control. Unlike HbA1c, which provides an average glucose level over 90 days, time in range gives a comprehensive picture of a person’s daily blood glucose control.6
Targets for time in range are agreed with the individual patient, but are typically above 4mmol/l, and below 7mmol/l before a meal and 8.5mmol/l after a meal.11 For most people with type I diabetes, a time in range above 70% is recommended (approximately 17 hours of a 24-hour day).12
The goal of diabetes management is to maximise the time in range and minimise high and low blood glucose levels to prevent the development of diabetes complications.6
Failure to maintain glycaemic control can lead to short-term complications
The short-term complications of poor glycaemic control are hypoglycaemia and hyperglycaemia.13–15
The dips (hypoglycaemia) and spikes (hyperglycaemia) in blood glucose have different causes and symptoms. If left untreated, hypo- and hyperglycaemia can lead to long-term complications and, in some cases, can be life-threatening.13,14
Long-term complications can arise from inadequate glycaemic control16,17









Stroke
Damage or blockage of blood vessels in the brain can cause stroke

Eye problems
Damage to blood vessels in the eyes can cause retinopathy
Heart disease
Poor blood supply due to narrowing or blockage of blood vessels can lead to myocardial infarction

Kidney disease
High blood glucose levels can cause nephropathy, potentially leading to kidney failure
Foot problems
Damage to the nerves of the feet can lead to foot ulcers and infections

Neuropathy
Consistently high blood glucose levels can lead to nerve damage
Every week in the UK, the impact of uncontrolled diabetes leads to:18
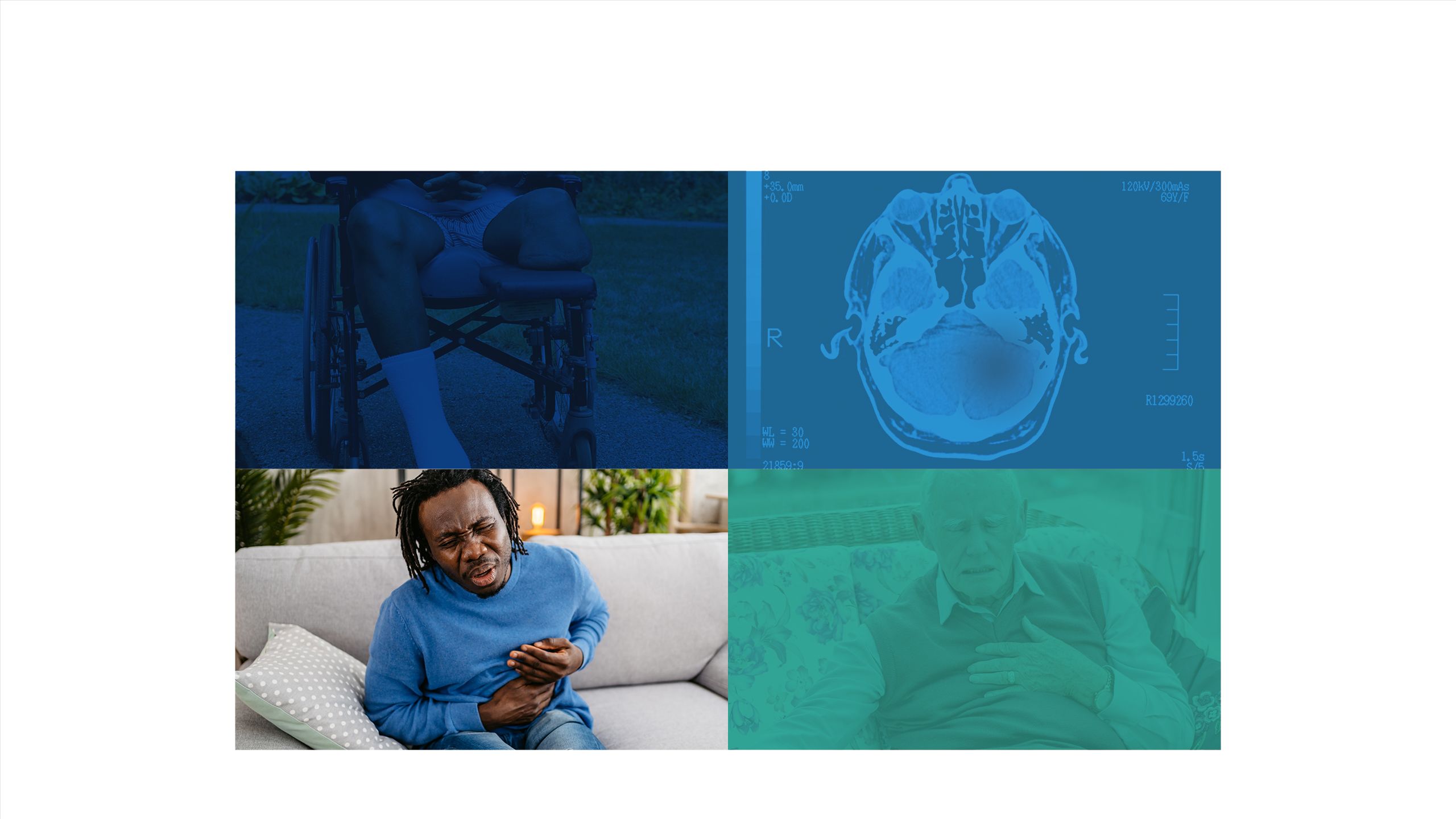

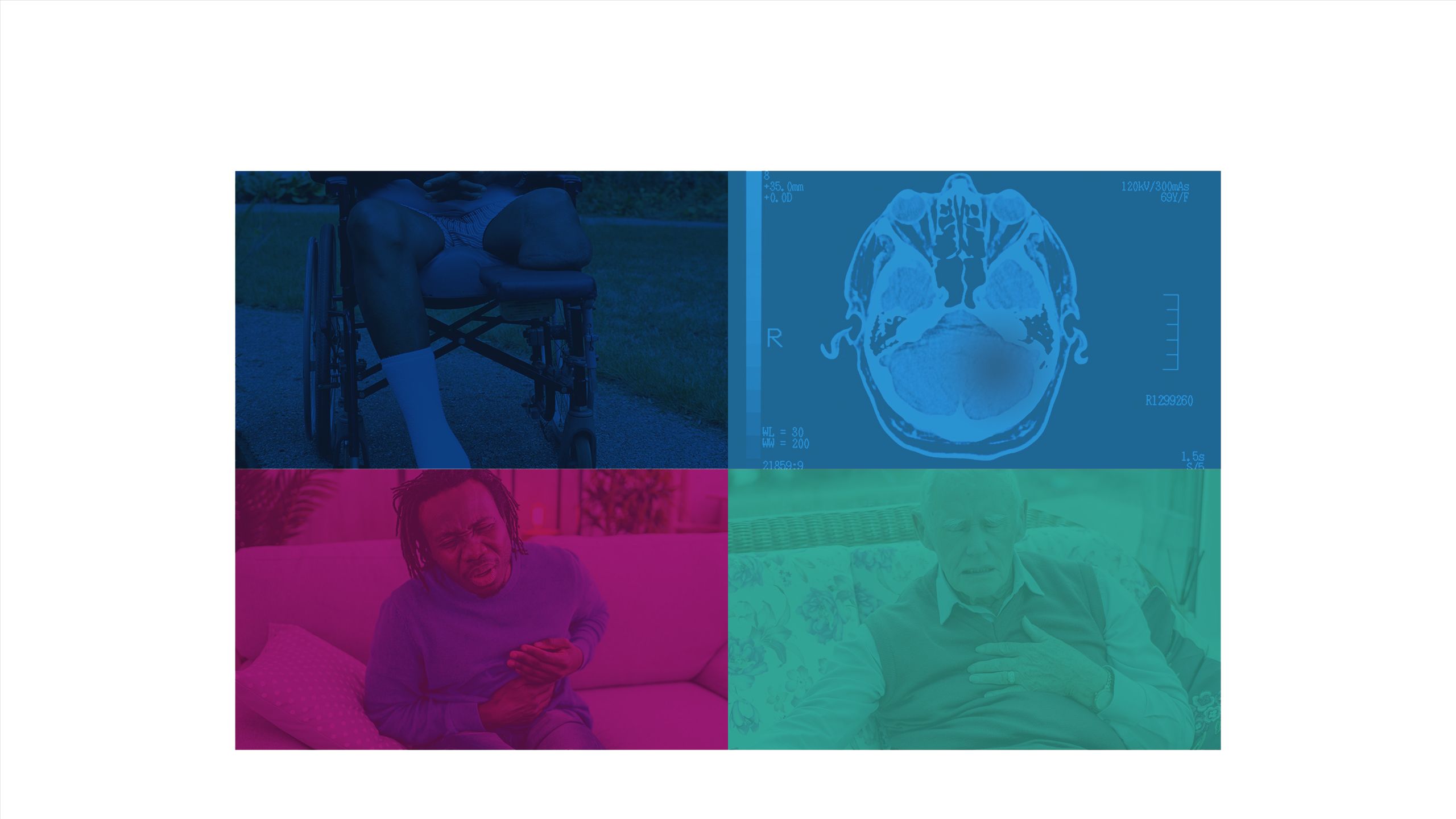
184 amputations
>770 strokes
590 heart attacks
2,300 cases of heart failure
Beyond the physical impact, diabetes also affects mental health
Patients with type I diabetes have an increased risk of poor mental health, particularly depression and anxiety.19
Type I diabetes also impacts:8,20
• Sleep
• Work life
• Parenting
• Psychosocial development
• Education
• Relationships

When patients are actively engaged in self-management, it can lead to…







A reduced risk of complications21
Improvements in glycaemic control21
Better quality of life21
Encouraging patients to monitor blood glucose and adhere to medication regimens can contribute to diabetes management and more importantly, improve overall health.22
The increasing role of technology in diabetes management

Historically, diabetes monitoring posed a challenge for both patients and healthcare providers23
The initial evolution of glucose monitoring:24,25
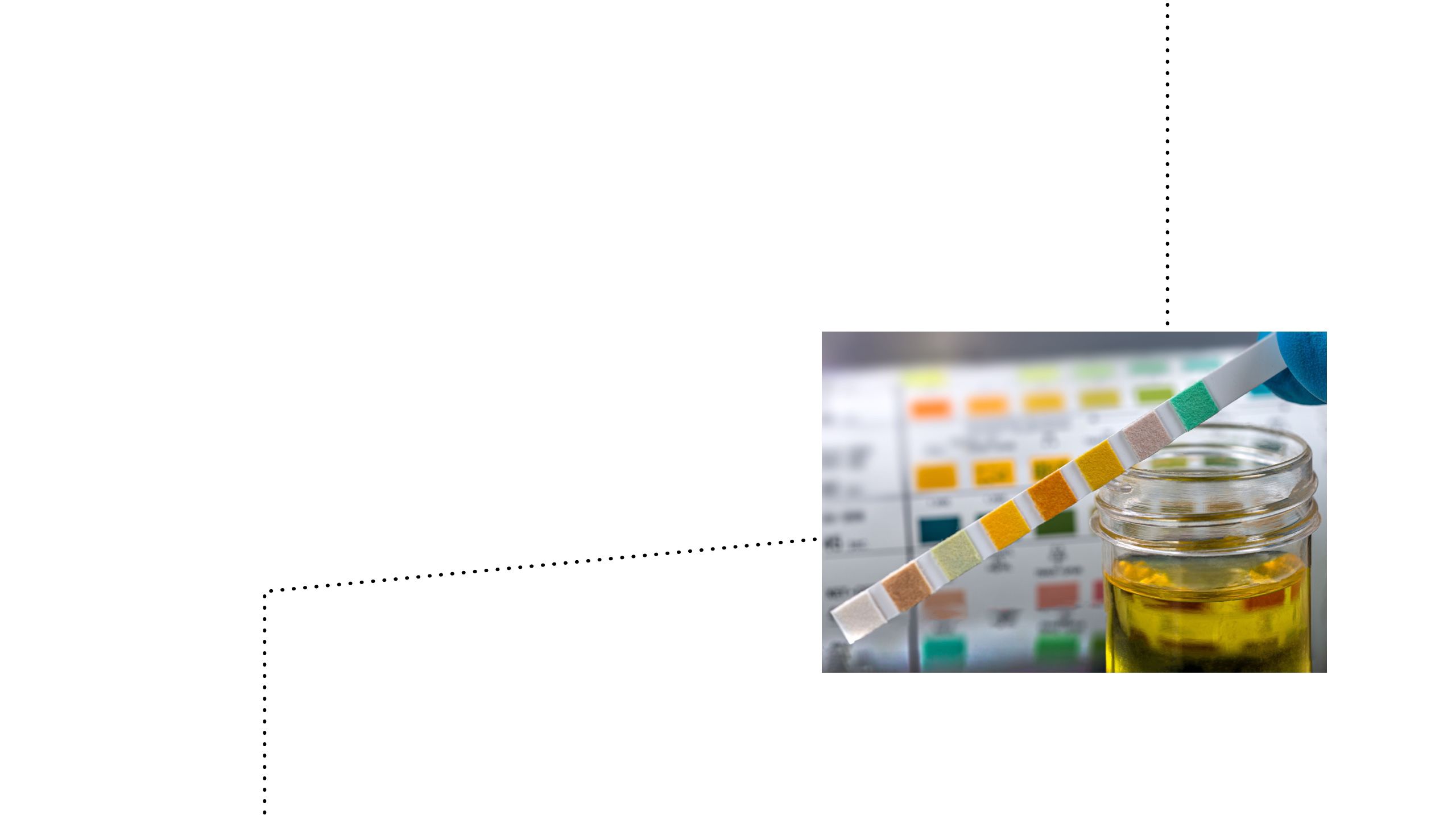
Urine glucose testing24
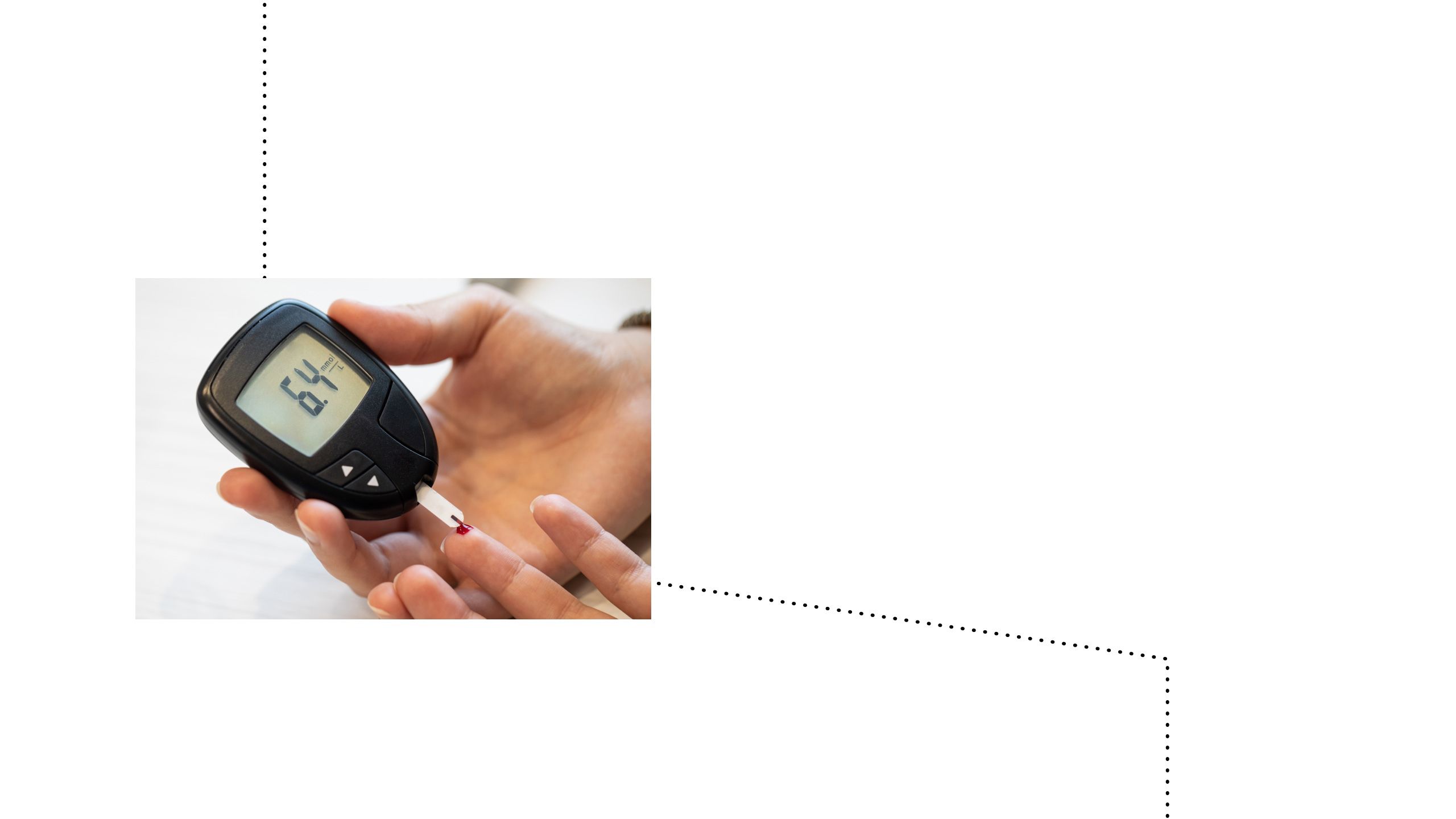
Finger-prick blood glucose tests24

The first continuous glucose monitoring system (regular finger-prick blood glucose samples over 3 days)25
Managing glucose levels represents a significant mental load for people living with type I diabetes, and can affect home life, education, training or work.8
Recent technological advancements have revolutionised diabetes management, improving patient outcomes26

Advances in diabetes technology have allowed patients with diabetes to achieve:27

Improved glycaemic control

Enhanced convenience

Reduced burden of disease

Improved quality of life


Technological tools empower patients to adopt a proactive and personalised approach to self-management, marking a significant shift towards effective and patient-centric diabetes care.27
What diabetes technologies are available for patients?

Several diabetes technologies are currently available to monitor and maintain glycaemic control
Continuous glucose monitoring (CGM)
Real-time CGM28
Measures and displays glucose levels continuously
Flash monitoring or intermittently scanned CGM28
Measures glucose levels continuously but requires scanning for visualisation and storage of glucose values
Professional CGM28
Placed on the person with diabetes in the healthcare professional's office and worn for 7 to 14 days. Data may be blinded or visible to the person wearing the device. The data are used to assess glycaemic patterns and trends




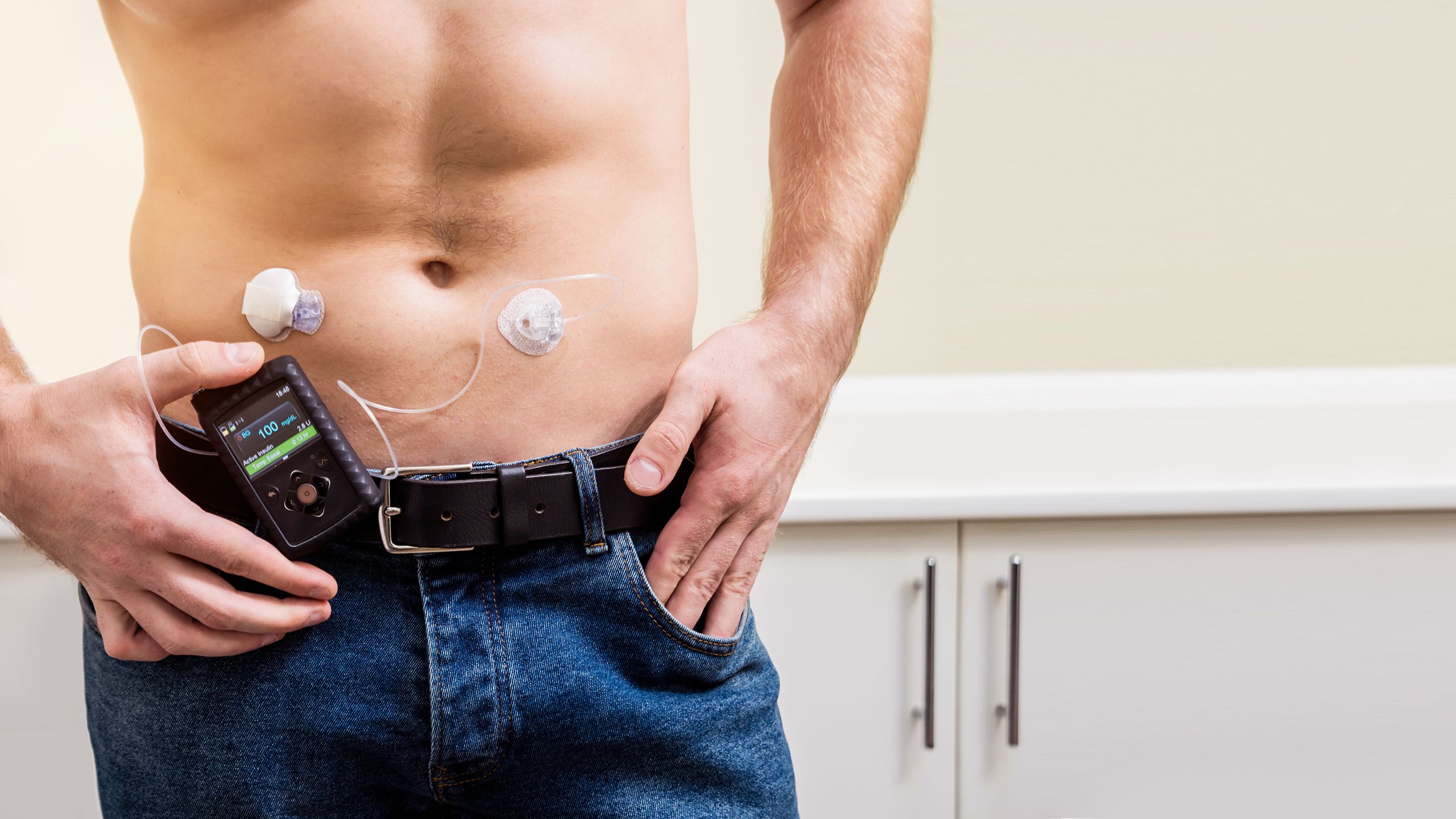
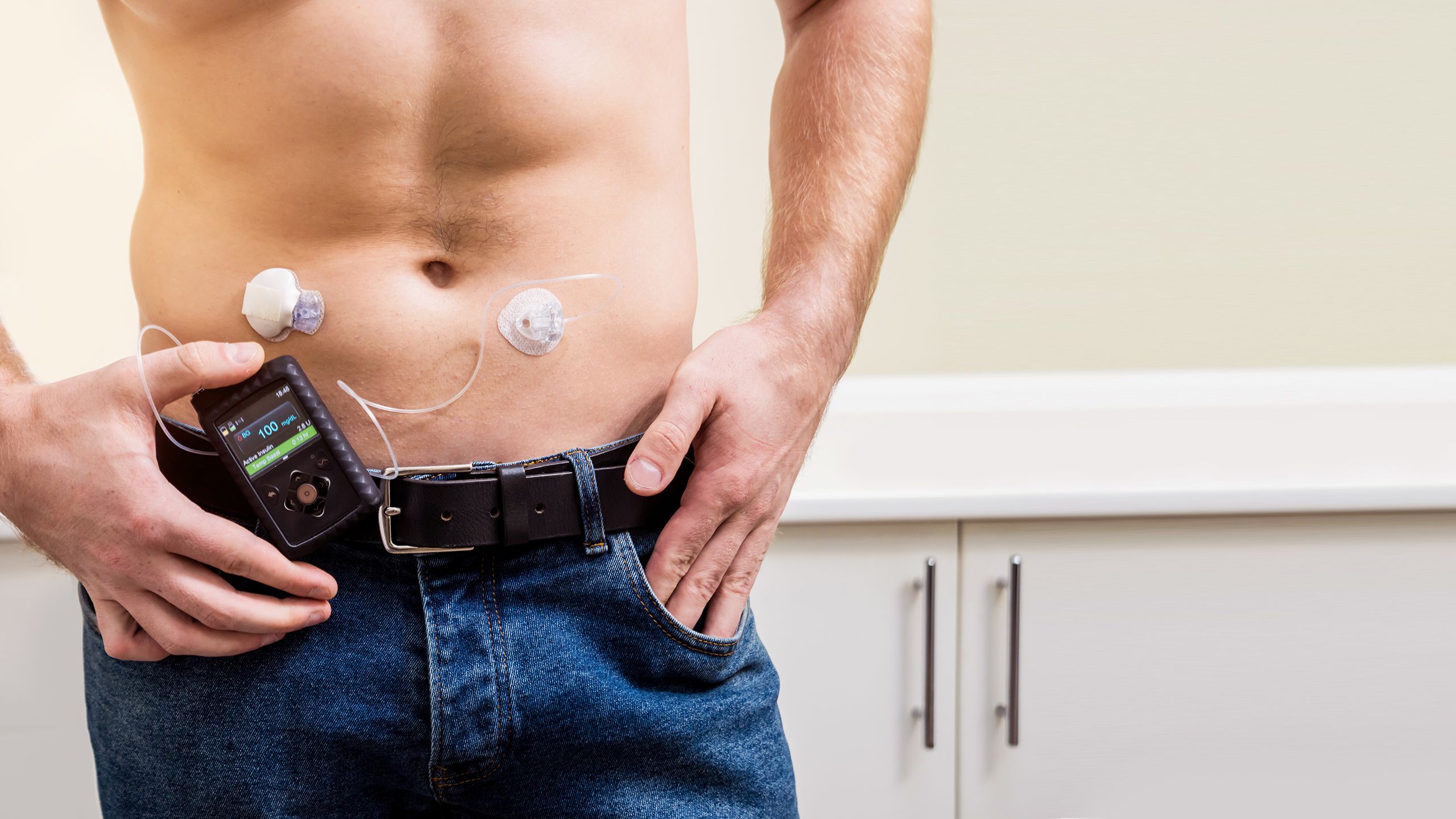
Real-time CGM
Patients can check their glucose levels and time in range at any time with CGM. It allows patients and the healthcare team to see:29
• If glucose levels are going up or down (indicating hypo- or hyperglycaemia)
• How glucose levels change over time
• What happens to glucose levels when the patient is asleep
A CGM is made up of:29
- Sensor: a small device attached to the arm or abdomen of the patient that senses how much glucose is in the interstitial fluid under the skin
- Reader: this shows the results sent by the sensor every few minutes (the results can also be sent to a smartphone)
Flash monitoring
A flash glucose monitor operates much like a real-time CGM, but with one key distinction: instead of glucose readings appearing automatically on a reader or smartphone, the patient needs to manually scan the sensor over a reader to obtain the readings. In contrast, a real-time CGM displays the latest glucose levels automatically, transmitted via Bluetooth.30
Another distinction lies in the placement of sensors. Flash monitoring requires sensors to be worn exclusively on the arms. In contrast, real-time CGM allows for sensor placement on various body areas, including the abdomen.30
Other diabetes technologies
Smart insulin pens
(also known as connected pens)
Smart insulin pens are reusable devices that automatically record when a patient has injected insulin, including time of injection and injection volume.31
The device also shows the amount of time that has passed since the last insulin dose so that patients can be alerted when a dose has been missed.31
Smart insulin pens can also connect with apps that keep insulin dosage and blood glucose data (for example, from CGM or flash monitoring) in one place. The patient can share the data with the healthcare team.31
Continuous subcutaneous insulin infusion
(insulin pumps)
An insulin pump is a small electronic device attached to the patient's body that releases regular insulin throughout the day and night to keep glucose levels within target range, acting like background or basal insulin, and avoiding the need for insulin injections.32
The healthcare team sets up the patient’s insulin rates, which can be adjusted based on the patient’s activity levels and meals. Patients can also give themselves a bolus dose when they eat, or a correction dose when the blood glucose level is too high. The pump calculates the dosage when the patient enters the amount of carbohydrates they plan on eating and their blood glucose level.32
There are 2 types of insulin pumps:32
| Type of insulin pump | Description |
| Tethered pump |
|
| Patch pump |
|
The cannula or patch pump needs to be changed every 2 or 3 days and moved to a different place on the body to prevent lipohypertrophy and itching/rashes.32
Closed-loop systems
(artificial pancreas)
An insulin pump and a CGM are connected to form a closed-loop system to maintain glycaemic control. The doses of insulin the patient needs are released via the pump, and some of these doses are adjusted automatically in response to the blood glucose levels that are continuously monitored via CGM.33
Hybrid closed-loop systems are regulated and available for patients to buy.33
There are 3 parts to a hybrid closed-loop system:33
| Parts of a hybrid closed-loop system | Description |
| CGM |
|
| An insulin pump |
|
| The algorithm |
|
In addition to device-based diabetes technologies, there are also a variety of websites, apps and services to help patients maintain blood glucose levels within the target range
|
Name of website/ app/service |
Description |
|
DigiBete link |
|
|
Low Carb Programme link |
|
|
MyType1 Diabetes link |
|
|
NHS Digital Weight Management Programme link |
|
NICE guidance on diabetes technologies

The National Institute for Health and Care Excellence (NICE)
has published guidance on the use of diabetes technology in patients with diabetes.7,34–37
The NICE guidance covers important information such as:7,34,35

Technology appraisal guidance has been published for continuous subcutaneous infusion (insulin pump therapy) and hybrid closed loop systems.10,37 Information on CGM devices is covered in NICE guidance.7,34–37
Continuous glucose monitoring and the NICE guidelines
Based on emerging clinical evidence, recommendations for CGM use have been updated across NICE guidance in diabetes management, including:
Which patients should be offered CGM?
- All adults with type I diabetes7
- All children and young people with type I diabetes34
- Children with type II diabetes who:34
- Have a need, condition or disability that means they cannot engage in monitoring their glucose levels by capillary blood glucose monitoring
- Would otherwise need to self-monitor at least 8 times a day
- Have recurrent or severe hypoglycaemia
- Adults with type II diabetes on multiple daily insulin injections who:35
- Have recurrent or severe hypoglycaemia
- Have impaired hypoglycaemia awareness
- Have a condition or disability that means they cannot self-monitor their blood glucose by capillary blood glucose monitoring
- Would otherwise need to self-monitor at least 8 times a day
- All pregnant people with type I diabetes36
- Pregnant people on insulin therapy who do not have type I diabetes but do have:36
- Problematic severe hypoglycaemia (with or without impaired hypoglycaemia awareness)
- Unstable blood glucose levels that are causing concern despite efforts to optimise glycaemic control
Gold score or Clarke score is recommended to quantify hypoglycaemia awareness in adults with type I diabetes.7 However, NICE guidance for type II diabetes does not recommend specific methods for assessing hypoglycaemia awareness.34
CGM use in diabetic patient populations: what is the evidence?
Type I adults
Both real-time and flash CGM provide clinical benefits over standard self-monitoring of blood glucose in key outcomes such as HbA1c, time in range and severe or nocturnal hypoglycaemia.7
Children with diabetes
Real-time CGM led to decreased HbA1c and increased time in range. Flash CGM had no clinically meaningful effect on any of the outcomes looked at in the evidence; however, it may be offered if real-time CGM is contraindicated or there is a clear preference for flash CGM.34
Type II adults
Flash CGM is recommended by NICE for adults with insulin-treated type II diabetes as a cost-effective option associated with good outcomes in clinical practice. However, real-time CGM is not recommended as there is no evidence that this technology is cost effective in this population.35
Pregnant people with diabetes
Compared with flash CGM, real-time CGM resulted in more patients achieving their glucose targets, fewer caesarean sections and fewer neonatal intensive care unit (NICU) admissions. Though there is more evidence for this technology, flash CGM may be offered if patients are unable to use real-time CGM or express a clear preference for flash CGM.36
General NICE guidance for CGM use
Provide CGM as part of diabetes self-management support by a team with expertise in its use.7,34–36
Advise CGM users to still take capillary blood glucose measurements to check their CGM device, and as a backup during rapid changes or device issues.7,34,35
Offer capillary blood glucose monitoring if a person declines or cannot use CGM.7,34,35
Integrate CGM education into ongoing programs for individuals with diabetes and/or their caregivers.7,34–36
Regularly review CGM use as part of diabetes care plans, emphasising the importance of consistent device wear.7,34,35
Address concerns about CGM usage by identifying and resolving problems, offering additional education, and providing emotional support.7,34,35
Tackle inequalities in CGM access by monitoring usage, identifying eligible groups with low uptake, and actively engaging them to consider CGM.7,34,35


Choosing a CGM device
The best device for each person can vary, which is why NICE guidance highlights the importance of providing choice of CGM based on individual preferences, needs, characteristics and the functionality of the devices available.7,34
Patient-specific factors7,34
• Fear, frequency, awareness and severity of hypoglycaemia
• Psychosocial factors
• Whether the device will affect the person's ability to do their job
• How unpredictable the person's activity and blood glucose levels are and whether erratic blood glucose is affecting their quality of life
• Whether the person has situations when symptoms of hypoglycaemia cannot be communicated or can be confused (eg, during exercise)
• Clinical factors that may make devices easier or harder to use
• Sensitivities to the device, eg, local skin reactions
• Body image concerns
Device-specific factors7,34
• Accuracy of the device
• Whether the device provides predictive alerts or alarms and if these need to be shared with anyone else (eg, a carer)
• Whether using the device requires access to particular technologies (such as a smartphone and up-to-date phone software)
• How easy the device is to use and take readings from, including for children or people with limited dexterity
• The person's insulin regimen or type of insulin pump, if relevant (taking into account whether a particular device integrates with their pump as part of a hybrid closed loop or insulin suspend function)
• Whether, how often and how the device needs to be calibrated, and how easy it is for the person to do this themselves
• How data can be collected, compatibility of the device with other technology, and whether data can be shared with the person's healthcare provider to help inform treatment
• Frequency of sensor replacement
Which patients should be offered continuous subcutaneous insulin infusion?

Adults and children 12 years and older37
- Patients who experience disabling hypoglycaemia resulting from attempts to achieve target HbA1c with multiple-daily insulin injection therapy
- Where HbA1c levels have remained high (69 mmol/mol [8.5%] or above) on multiple-daily insulin injection therapy despite a high level of care

Children younger than 12 years37
- Where multiple-daily insulin injection therapy is considered to be impractical or inappropriate.
Technology appraisal guidance has been published for continuous subcutaneous infusion (insulin pump therapy) and hybrid closed loop systems.8,37 Information on CGM devices is covered in NICE guidance.7,34–37
Which patients should be offered hybrid closed loop systems?
Hybrid closed loop (HCL) systems are recommended as an option for managing blood glucose levels in type I diabetes for the following individuals:8
- Children and young people
- Women, trans men and non-binary people who are pregnant or planning to become pregnant
- Adults who have an HbA1c of 58 mmol/mol (7.5%) or more, or have disabling hypoglycaemia, despite best possible management with at least one of the following:
- Continuous subcutaneous insulin infusion
- Real-time CGM
- Intermittently scanned ‘flash’ CGM
Hybrid closed loop (HCL) systems must only be used if:8

Conclusion

Achieving optimal glycaemic control is paramount in mitigating both short- and long-term complications associated with diabetes. However, the relentless task of managing blood glucose levels poses a considerable mental burden for patients and their caregivers alike. Fortunately, advancements in diabetes technology, including continuous glucose monitoring, insulin pumps and hybrid closed-loop systems, offer a promising solution.
Recent NICE guidance is expected to broaden the use of diabetes technologies across the UK. This may help to improve the lives of patients with diabetes by providing them with better tools for managing their condition, reducing the risk of complications, and empowering them to take control of their health.
Useful links
Follow the links below for more information and resources about diabetes technologies and diabetes available on the MIMS website.
MIMS Visual guide to diabetes devices
References

1. Diabetes UK. How many people in the UK have diabetes? Last accessed February 2024.
2. Diabetes UK. High quality care for children and young people with type 1 diabetes. Last accessed February 2024.
3. Diabetes UK. 1-in-10 adults living with diabetes by 2030. October 2021.
4. Bin Rakhis SA Sr, AlDuwayhis NM, Aleid N, et al. Glycemic Control for Type 2 Diabetes Mellitus Patients: A Systematic Review. Cureus 2022; 14(6): e26180.
5. Diabetes UK. What is HbA1c? Last accessed February 2024.
6. Diabetes. Time in range. Last accessed February 2024.
7. NICE. Type 1 diabetes in adults: diagnosis and management. NG17. August 2022.
8. NICE. Hybrid closed loop systems for managing blood glucose levels in type 1 diabetes. TA943. Last accessed February 2024.
9. Diabetes UK. Hypoglycaemia (hypo). Last accessed February 2024.
10. Diabetes UK. Hyperglycaemia (hyper). Last accessed February 2024.
11. Diabetes UK. Time in range. Last accessed February 2024.
12. Endocrine Society. Time in range and diabetes. January 2022.
13. Diabetes. Short term complications of diabetes. October 2023.
14. NHS. Low blood sugar (hypoglycaemia). August 2023.
15. NHS. High blood sugar (hyperglycaemia). May 2022.
16. NICE CKS. Diabetes - type 1: What are the complications? July 2023.
17. NHS Inform. Type 1 diabetes. November 2023.
18. Diabetes. Number of people living with diabetes in the UK tops 5 million for the first time. Last accessed February 2024.
19. Ferrier L, Ski CF, O'Brien C, et al. Bridging the gap between diabetes care and mental health: Perspectives of the Mental health IN DiabeteS Optimal Health Program (MINDS OHP). BMC Endocr Disord 2021; 21(1): 96.
20. van Duinkerken E, Snoek FJ, de Wit M. The cognitive and psychological effects of living with type 1 diabetes: A narrative review. Diabet Med 2020; 37(4): 555–63.
21. Hamilton K, Stanton-Fay SH, Chadwick PM, et al. Sustained type 1 diabetes self-management: Specifying the behaviours involved and their influences. Diabet Med 2021; 38(5): e14430.
22. Bussell JK, Cha E, Grant YE, et al. Ways Health Care Providers Can Promote Better Medication Adherence. Clin Diabetes 2017; 35(3): 171–7.
23. Smith IP, Whichello CL, Veldwijk J, et al. Diabetes patient preferences for glucose-monitoring technologies: Results from a discrete choice experiment in Poland and the Netherlands. BMJ Open Diabetes Res Care 2023; 11(1): e003025.
24. Hirsch IB. Introduction: History of Glucose Monitoring. 2018. In: Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington (VA): American Diabetes Association.
25. Didyuk O, Econom N, Guardia A, et al. Continuous glucose monitoring devices: Past, present, and future focus on the history and evolution of technological innovation. J Diabetes Sci Technol 2021; 15(3): 676–83.
26. Zahid M, Dowlatshahi S, Kansara AH, et al. The Evolution of Diabetes Technology - Options Toward Personalized Care. Endocr Pract 2023; 29(8): 653–62.
27. Alcántara-Aragón V. Improving patient self-care using diabetes technologies. Ther Adv Endocrinol Metab 2019; 10: 2042018818824215.
28. American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Care in Diabetes-2024. Diabetes Care 2024; 47(Suppl 1): S126–44.
29. NHS. Continuous glucose monitoring (CGM) and flash). July 2021.
30. Diabetes UK. Flash glucose monitors (freestyle libre) and continuous glucose monitors (CGM). Last accessed February 2024.
31. Diabetes UK. Smart insulin pens: How they work, and your experiences of using them. Last accessed February 2024.
32. Diabetes UK. Insulin pumps. Last accessed February 2024.
33. Diabetes UK. Closed loop systems (artificial pancreas). Last accessed February 2024.
34. NICE. Diabetes (type 1 and type 2) in children and young people: diagnosis and management. NG18. May 2023.
35. NICE. Type 2 diabetes in adults: management. NG28. June 2022.
36. NICE. Diabetes in pregnancy: management from preconception to the postnatal period. NG3. December 2020.
37. NICE. Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus. TA151. July 2008.
Published by Haymarket Media Group Ltd, Bridge House, 69 London Road, Twickenham, TW1 3SP
You acknowledge that whilst Haymarket endeavours to ensure that information on this site is accurate and complete at the time of first publication, it is provided only for general information, is not intended to address your particular requirements and does not constitute any form of advice or recommendation by Haymarket. You acknowledge that the information on this site does not necessarily reflect the views and opinions of Haymarket, that such information should not be relied upon by you in making (or refraining from making) any specific investment or other business, professional or personal decisions and that you should seek independent professional advice before making any such decision. You also acknowledge that views and opinions expressed in content submitted by third party contributors are views and opinions of the authors of that content and not Haymarket’s views and opinions.
©2024 Haymarket Media Group Ltd.



